Our research agenda in quantum biology builds on the pioneering works of Linus Pauling and his contemporaries at the intersection of quantum physics and molecular biology. Pauling won the Nobel Prize in Chemistry in 1954 for his groundbreaking research on chemical bonds and molecular structures. His journey began in 1926 with a fellowship to conduct quantum research under the supervision of Arnold Sommerfeld, Niels Bohr, and Erwin Schrödinger. In the 1930s, Pauling described chemical bonding as a quantum superposition phenomenon, which played a crucial role in his participation in the discovery of alpha helix and beta sheet structures of proteins.
We focus on using the tools of modern quantum information science to deepen our understanding of biochemical phenomena. Recent developments in this field have revealed that superpositions shared in composite systems, namely quantum correlations known as entanglement and discord, can serve as valuable resources in quantum technologies. An intriguing possibility that our research explores is whether evolution may have discovered how to create, maintain, and control quantum correlations before us. Whatever the correct answer, it could further our understanding of biochemical phenomena and pave the way for a new program on bio-inspired quantum technologies.
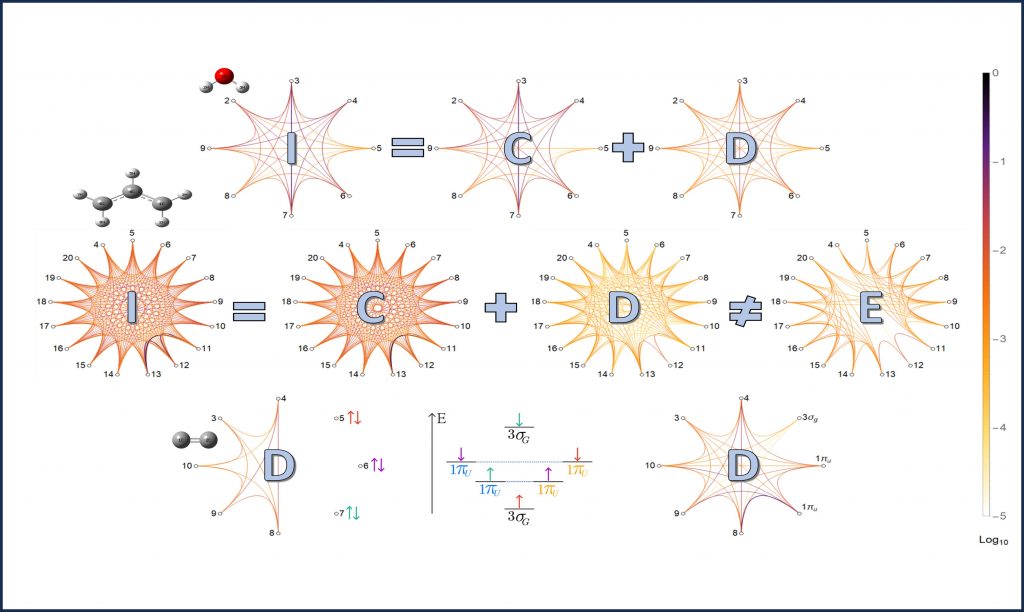
Did nature harness quantum resources to give birth to living systems, or were these resources purposely excluded to make life feasible? To address these questions, we can explore different many-body systems. Our focus lies on the electronic states of molecules. The delocalization of electrons through covalent bonds creates fermionic mode entanglement and discord between molecular orbitals while stabilizing bonded atoms and molecules by a certain amount of energy. To quantify classical and quantum orbital correlations, we have developed a methodology compatible with all conventional quantum chemistry algorithms and software and tested it on some prototypical molecules [J.6]. In our research, we aim to investigate the dynamical behavior of these orbital correlations and interrogate their contributions to the physical, chemical, and biological properties and functions of various π-conjugated molecules.
One of the biggest challenges in this research is the computational limits of quantum chemistry. To overcome this, we plan to deliver advances beyond the state of the art in quantum chemistry together with our collaborators. We use cutting-edge numerical methods based on new tools from quantum computation, condensed matter theory, and computer engineering to describe the electronic states of strongly correlated molecules and complexes.
Similar to how covalent bonds gain stability through electron delocalization, hydrogen bonds can also be stabilized through proton delocalization. Exploring this possibility was one of the primary objectives of Dr. Onur Pusuluk’s Ph.D. research [J.1, J.2, J.3]. Investigating the quantum coherence and correlations arising within hydrogen bond networks and their contributions to the physical, chemical, and biological properties and functions of molecules is another part of our research agenda. To achieve this, we intend to push the boundaries of post-Hartree-Fock methods, which typically account only for electron delocalization while fixing all proton positions.
Our proposed research in quantum biology also aims to investigate the role of molecular correlations in nonequilibrium biochemical processes using modern techniques of quantum thermodynamics. These techniques are at the forefront of current practice in molecular biology and include single-shot quantum thermodynamics and its resource theory [J.7], an application of quantum information theory to study small and strongly correlated thermodynamical systems that fall outside the scope of traditional statistical treatments. Additionally, it characterizes physical systems based on the amount of control an experimenter can exert on them.
Our research focus on applying the thermomajorization criteria to computational biophysics problems. These criteria correspond to a set of necessary and sufficient conditions for out-of-equilibrium thermodynamic state transformations of microscopic, quantum, or highly correlated systems.
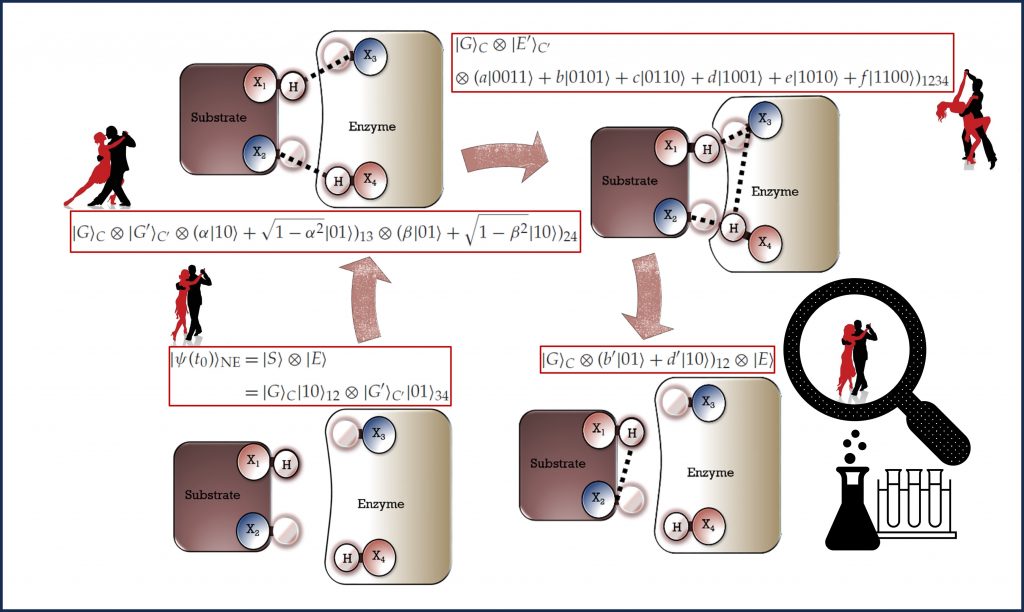
While imposing stricter constraints on thermodynamic transitions than the traditional second law of thermodynamics, the thermomajorization criteria also permit quantum correlations to drive an otherwise impossible biochemical reaction without being consumed. The possibility for a catalytic role of orbital correlations, which we first introduced in [U.3], warrants further investigation, and its verification may significantly change our understanding of enzyme catalysis.
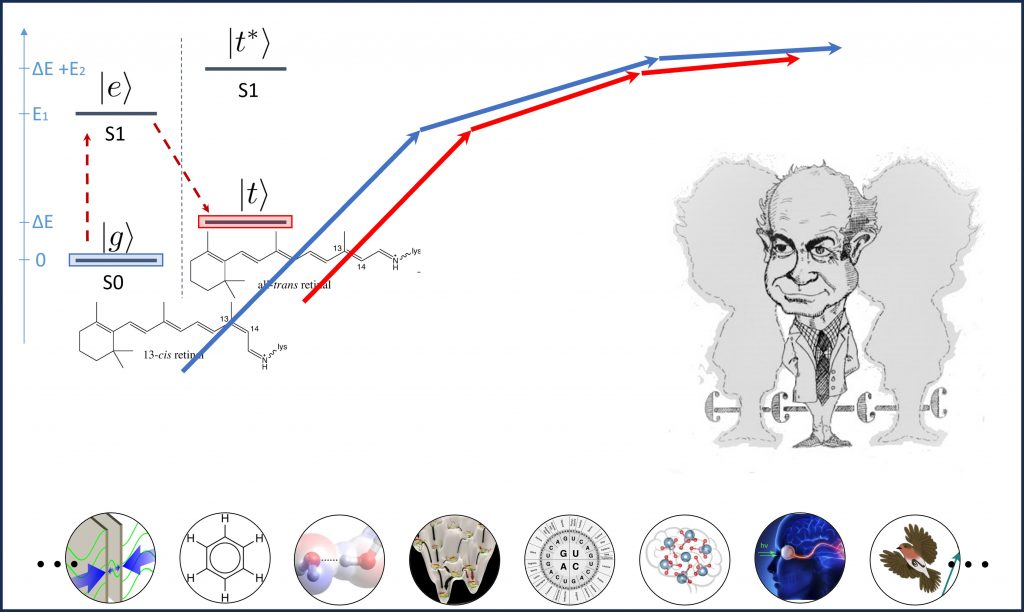
Moreover, this research methodology holds immense potential in shedding new light on enduring debates in molecular biology. Through the application of this methodology, we have recently demonstrated that biomolecular correlations can boost the efficiency of photoisomerization of the retinal chromophore in rhodopsin [U.6].
In addition to the theoretical and computational research mentioned above, the implementation of single-molecule experiments holds significant importance in revealing new discoveries by applying quantum information and thermodynamic principles to biology. In pursuit of this objective, we have recently proposed a theoretical model [U.3] indicating an enhancement in the efficiency of the photoisomerization process through the utilization of biomolecular correlations. We are planning to design experiments to test this theoretical model.
In a separate theoretical study, we are trying to demonstrate the possibility of steady-state entanglement between two molecules through Casimir-Polder interaction. We aspire to experimentally validate this prediction with our collaborators as well. The ultimate goal of this research is to uncover novel methods facilitating the control of molecular correlations, thereby enabling the integration of organic molecules into quantum technologies.
Furthermore, we are currently investigating the metrological advantages of molecular correlations in the formation of light-sensitive radical pairs during avian magnetoreception. We also plan to conduct single-molecule experiments in this domain.